|
NaNH2 - Part 3
|
In the reaction we want to investigate (Na + NH3 → NaNH2 + 1/2 H2), metallic Na, ionic NaNH2 and H2 are involved together with ammonia.
In this section, we want to perform the same kind of calculations considered for solid NH3, analyzing differences in CRYSTAL input and their one-electron properties and also calculate the energy of H2 molecule.
Once obtained all the needed data, we will calculate the enery of the reaction.
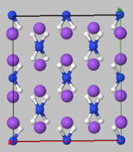
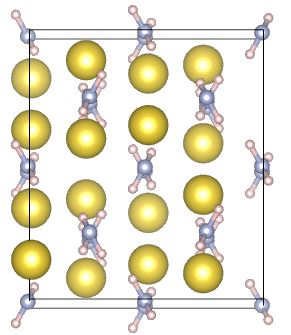
- Periodic systems and their properties - Metallic Na and solid NaNH2 cases
- Calculation of reaction energy
Periodic systems and their properties - Metallic Na and solid NaNH2 cases
| Na: metal | NaNH2: ionic crystal |
 |
 |
i) CRYSTAL input for metallic sodium and sodium amide
Below CRYSTAL inputs for Na and NaNH2 are reported.
METALLIC SODIUM
CRYSTAL
0 0 0
229
4.1297
1
11 0 0 0
END
11 11
0 0 7 2. 1.
2.60411099270E+04 6.18063428110E-04
3.90612685480E+03 4.77486044140E-03
8.88974549930E+02 2.44716848290E-02
2.51454979610E+02 9.47553949770E-02
8.16501435120E+01 2.68674969200E-01
2.89041584010E+01 4.79254754400E-01
1.06257829320E+01 3.32485914690E-01
0 0 3 2. 1.
5.37694101790E+01 1.95277318720E-02
1.63082430250E+01 9.26480107940E-02
2.37303841250E+00 -3.99386701720E-01
0 0 2 1. 1.
9.57307726030E-01 1.64285953910E+00
4.08064609590E-01 5.56925969660E-01
0 0 1 0. 1. *
2.12721167582E-01 9.99992062637E-01
0 0 1 0. 1. *
7.82096876345E-02 9.99700925116E-01
0 2 5 6. 1.
1.38079799890E+02 5.79518919290E-03
3.22327003930E+01 4.16208462510E-02
9.98160753600E+00 1.62819168850E-01
3.48220339280E+00 3.60117846470E-01
1.22991346200E+00 4.48589798890E-01
0 2 1 0. 1. *
4.03415531731E-01 1.00001437874E+00
0 2 1 0. 1. *
8.50539333916E-02 1.00010780928E+00
0 3 1 0. 1. *
2.60862497919E+00 1.00000000000E+00
0 3 1 0. 1. *
4.33739110192E-01 1.00000000000E+00
0 3 1 0. 1. *
1.11542851169E-01 1.00000000000E+00
99 0
END
DFT
PBE
END
TOLINTEG
8 8 8 8 16
TOLDEE
8
SHRINK
8 8
MULPOPAN
END
NaNH2
CRYSTAL
0 0 1
70
8.95227 9.77379 7.48905
3
11 0. 0.15037 1.45765E-17
7 0. 0. 0.25271
1 0.03386 0.07466 0.33959
END
11 11
0 0 7 2. 1.
2.60411099270E+04 6.18063428110E-04
3.90612685480E+03 4.77486044140E-03
8.88974549930E+02 2.44716848290E-02
2.51454979610E+02 9.47553949770E-02
8.16501435120E+01 2.68674969200E-01
2.89041584010E+01 4.79254754400E-01
1.06257829320E+01 3.32485914690E-01
0 0 3 2. 1.
5.37694101790E+01 1.95277318720E-02
1.63082430250E+01 9.26480107940E-02
2.37303841250E+00 -3.99386701720E-01
0 0 2 1. 1.
9.57307726030E-01 1.64285953910E+00
4.08064609590E-01 5.56925969660E-01
0 0 1 0. 1. *
3.43569978393E-01 9.99992062637E-01
0 0 1 0. 1. *
8.28104267544E-02 9.99700925116E-01
0 2 5 6. 1.
1.38079799890E+02 5.79518919290E-03
3.22327003930E+01 4.16208462510E-02
9.98160753600E+00 1.62819168850E-01
3.48220339280E+00 3.60117846470E-01
1.22991346200E+00 4.48589798890E-01
0 2 1 0. 1. *
4.02257637589E-01 1.00001437874E+00
0 2 1 0. 1. *
9.81092534535E-02 1.00010780928E+00
0 3 1 0. 1. *
2.60738659094E+00 1.00000000000E+00
0 3 1 0. 1. *
4.03951011422E-01 1.00000000000E+00
0 3 1 0. 1. *
9.84682729829E-02 1.00000000000E+00
7 4
0 0 6 2. 1.
0.417351E+04 0.183477D-02
0.627458E+03 0.139946D-01
0.142902E+03 0.685866D-01
0.402343E+02 0.232241E+00
0.128202E+02 0.469070E+00
0.439044E+01 0.360455E+00
0 1 3 5. 1.
0.116264E+02 -.114961E+00 0.675797D-01
0.271628E+01 -.169117E+00 0.323907E+00
0.772218E+00 0.114585E+01 0.740895E+00
0 1 1 0. 1.
0.212031E+00 0.100000E+01 0.100000E+01
0 3 1 0. 1.
0.800000E+00 0.100000E+01
1 3
0 0 3 1.0 1.0
.1873113696D+02 .3349460434D-01
.2825394365D+01 .2347269535D+00
.6401216923D+00 .8137573262D+00
0 0 1 0.0 1.0
.1612777588D+00 .1000000000D+01
0 2 1 0.0 1.0
.1100000000D+01 .1000000000D+01
99 0
END
DFT
PBE
END
TOLINTEG
8 8 8 8 16
TOLDEE
8
SHRINK
8 8
MULPOPAN
END
The chosen basis set for Na can be found at pages 12 and 13 of the following file.
Two new keywords appear in the Hamiltonian section of the current inputs: TOLINTEG and TOLDEE. They allow to control the tolerances on integrals and total energy calculations, respectively. In NaNH2 input, the three numbers following the keyword CRYSTAL are not all equal to zero as in all previous cases, but the last one is equal to 1 for shifting the origin of the cell.
Exercise:
As already done for solid NH3, basing on the provided sample inputs, study the effect of the functional and/or method on total energy, N and H Mulliken charges, band gap and overlap population (OVPOP) of the N-H bond.
ii) One-electron properties of Na and NaNH2
We intend to study properties of Na and NaNH2.
Below PROPERTIES inputs for band structure, density of states and electron charge density are reported for the two systems.
- Na
- BAND
Path: G-H-P-N-G
4 8 120 3 20 1 0
0 0 0 4 -4 4
4 -4 4 2 2 2
2 2 2 0 0 4
0 0 4 0 0 0
END - NEWK
12 16
1 0
DOSS
4 200 3 20 1 12 0
5 1 2 3 4 5
6 6 7 9 10 12 13
3 8 11 14
15 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29
END - ECHG
0
100
COORDINA
-4. -4. 0.0
4. -4. 0.0
4. 4. 0.0
MARGINS
2.0 2.0 2.0 2.0
END
PATO
0 0
ECHG
0
100
COORDINA
-4. -4. 0.0
4. -4. 0.0
4. 4. 0.0
MARGINS
2.0 2.0 2.0 2.0
END
END - NaNH2
- BAND
Path G Z Y T G
4 8 120 25 55 1 0
0 0 0 4 4 0
4 4 0 4 0 4
4 0 4 8 4 4
8 4 4 0 0 0
END - NEWK
8 8
1 0
DOSS
3 200 25 55 1 14 0
-1 1
-1 5
-1 9
END - NEWK
8 8
1 0
DOSS
4 200 25 55 1 14 0
10 1 2 3 4 5 117 118 122 173 174
12 6 7 9 10 12 13 119 120 123 124 175 176
6 8 11 14 121 125 177
20 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 126 127 128 129 130
END - For electron charge density, use the same input reported for Na.
Exercise:
Calculate band structure, density of states and electron charge density of Na and NaNH2 with all adopted functionals for the calculations of total energy, as for NH3. The input will always be the same but be careful to use the correct fort.9 file where wavefunction and Hamiltonian information are stored.
Which difference do you notice among the difference functional? And among the difference systems?
Calculation of reaction energy

Metallic sodium and ammonia react according to the following scheme, producing sodium amide and hydrogen:
Hydrogen
MOLECULE
1
2
1 0. 0. 0.
1 0. 0. 0.7414
OPTGEOM
END
END
1 3
0 0 3 1.0 1.0
.1873113696D+02 .3349460434D-01
.2825394365D+01 .2347269535D+00
.6401216923D+00 .8137573262D+00
0 0 1 0.0 1.0
.1612777588D+00 .1000000000D+01
0 2 1 0.0 1.0
.1100000000D+01 .1000000000D+01
99 0
END
DFT
SVWN
END
MULPOPAN
END
3-1p1G basis set is used as basis set for H as for NaNH2 and NH3 calculations.
Exercise:
Analyze again the effect of the functional/method on the final results as previously done, by filling a table where you report total energy, H Mulliken charge, band gap and overlap population (OVPOP) of the H-H bond. Use the same methods chosen for the other systems.
Now that we have computed the energy of all reagents and products of the considered reaction, it is possible to calculate the reaction energy at different levels of theory.
Exercise:
Consider the usual formula ΔE= ∑P EPνP - ∑R ERνR to calculate the reaction energy with all the functionals adopted for the calculation of the total energy of the involved systems.
Pay attention: you must refer to the total energy per unit formula!